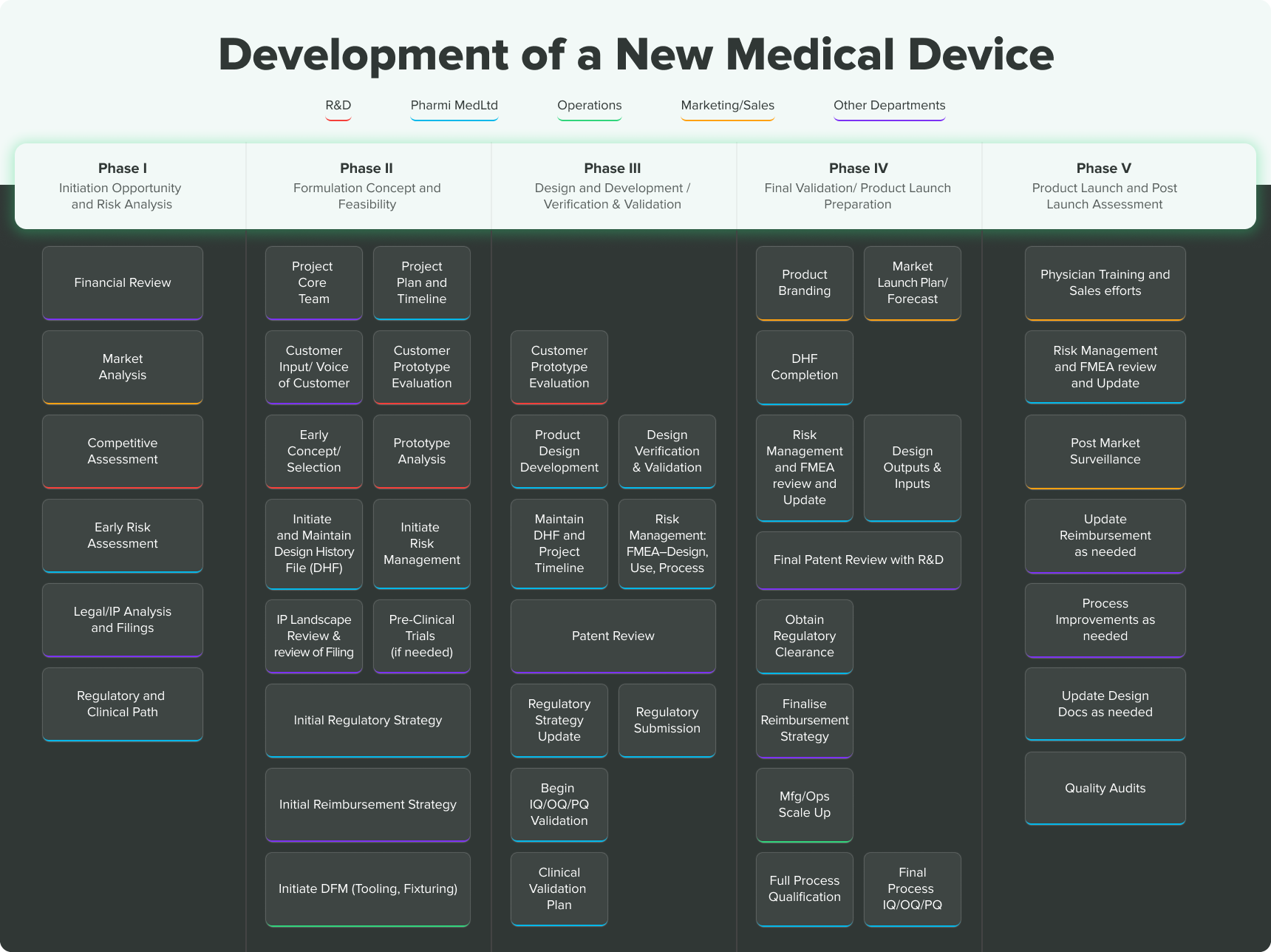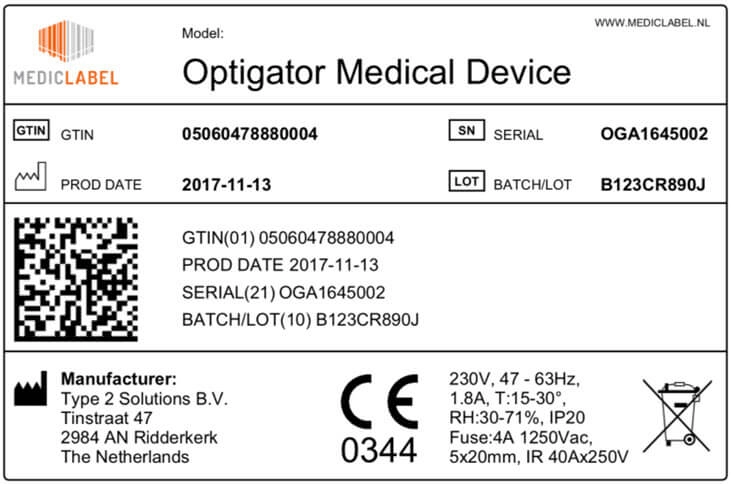
Frontiers | Medical Device Development Process, and Associated Risks and Legislative Aspects-Systematic Review
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine

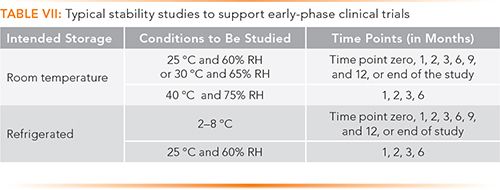
BS EN ISO 23640:2011 - In vitro diagnostic medical devices. Evaluation of stability of in vitro diagnostic reagents (British Standard)