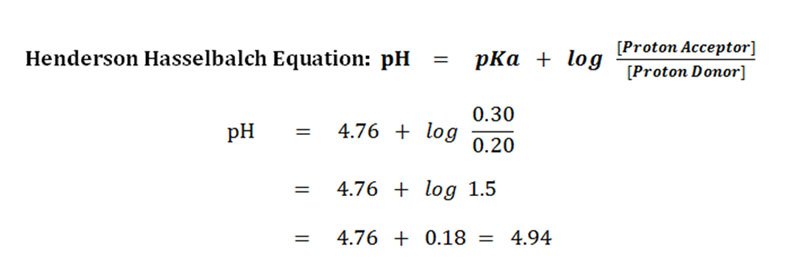
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]
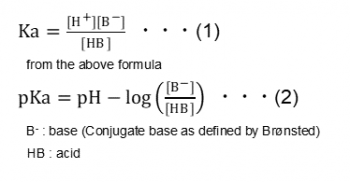
How should the acid dissociation constant pKa be measured? | Automatic Potentiometric Titrators | Faq | Kyoto Electronics Manufacturing Co.,Ltd.("KEM")
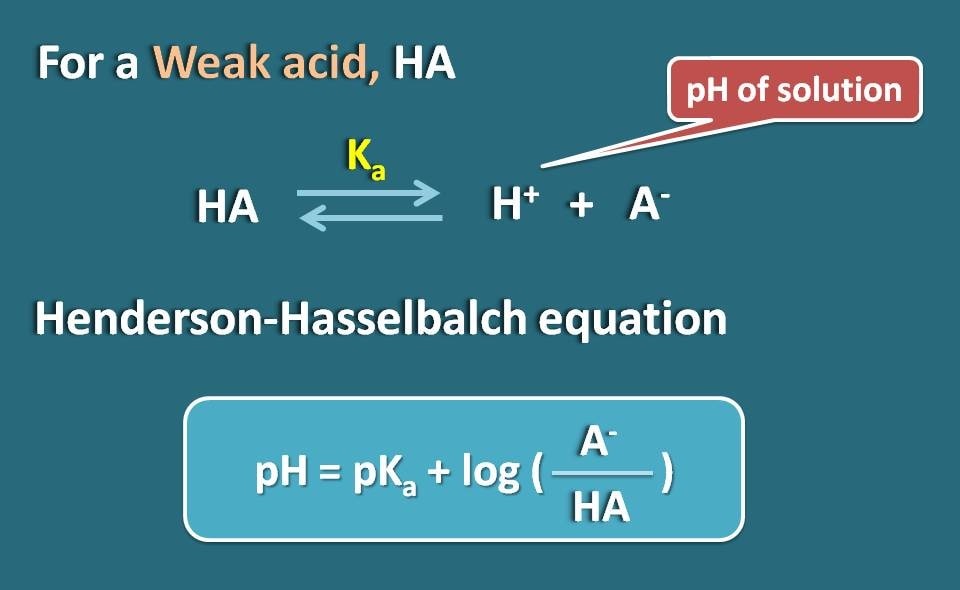
![SOLVED: Question #16: In buffer calculations, we used the Henderson-Hasselbalch equation. [A-] pH = pKa + logâ‚ â‚€ [THA] Suppose you are using a weak monobasic acid, HA, with a pKa of SOLVED: Question #16: In buffer calculations, we used the Henderson-Hasselbalch equation. [A-] pH = pKa + logâ‚ â‚€ [THA] Suppose you are using a weak monobasic acid, HA, with a pKa of](https://cdn.numerade.com/ask_previews/3d97130d-3610-4ac7-8aee-a2500b0f71f3_large.jpg)
SOLVED: Question #16: In buffer calculations, we used the Henderson-Hasselbalch equation. [A-] pH = pKa + logâ‚ â‚€ [THA] Suppose you are using a weak monobasic acid, HA, with a pKa of

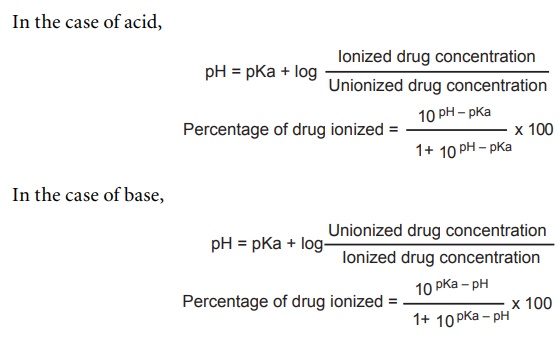








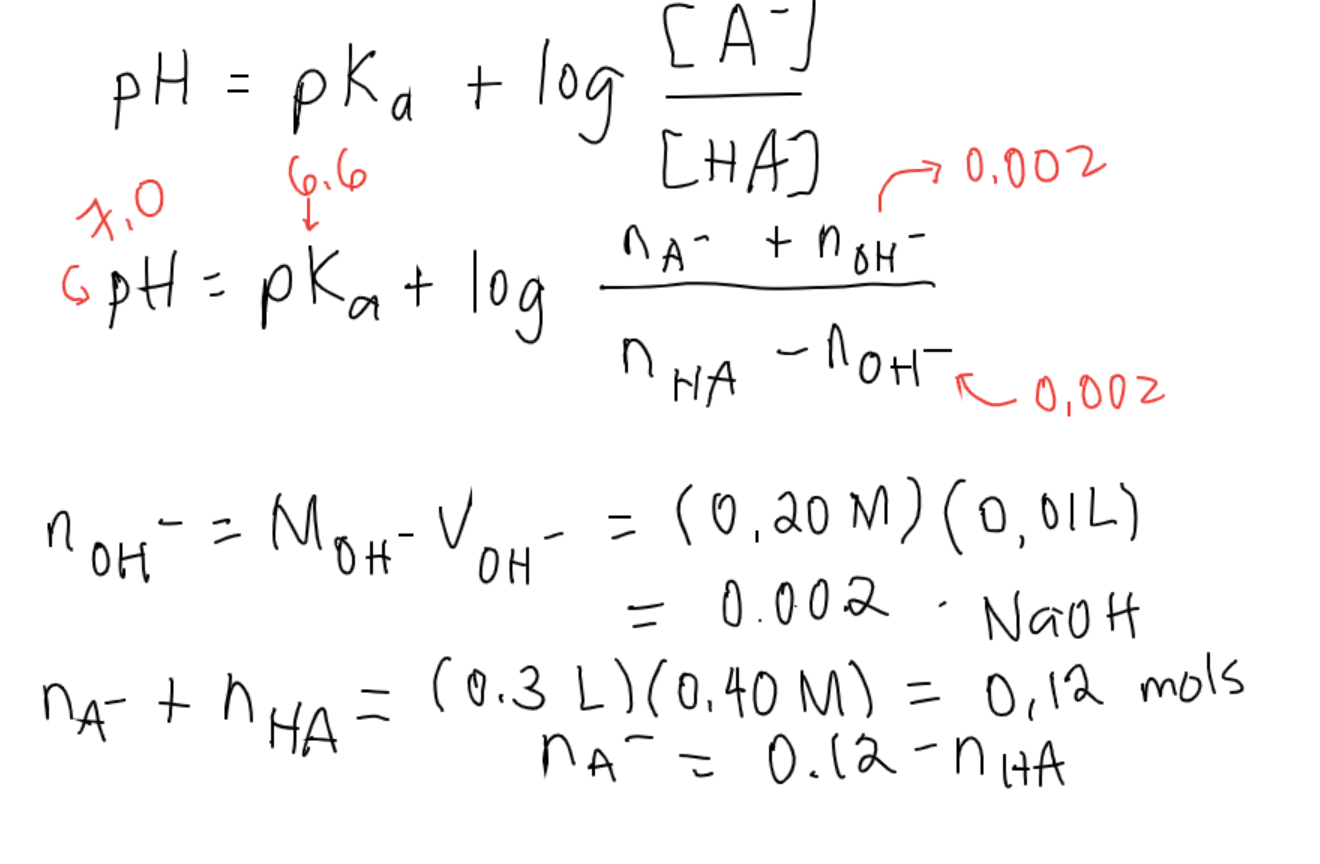
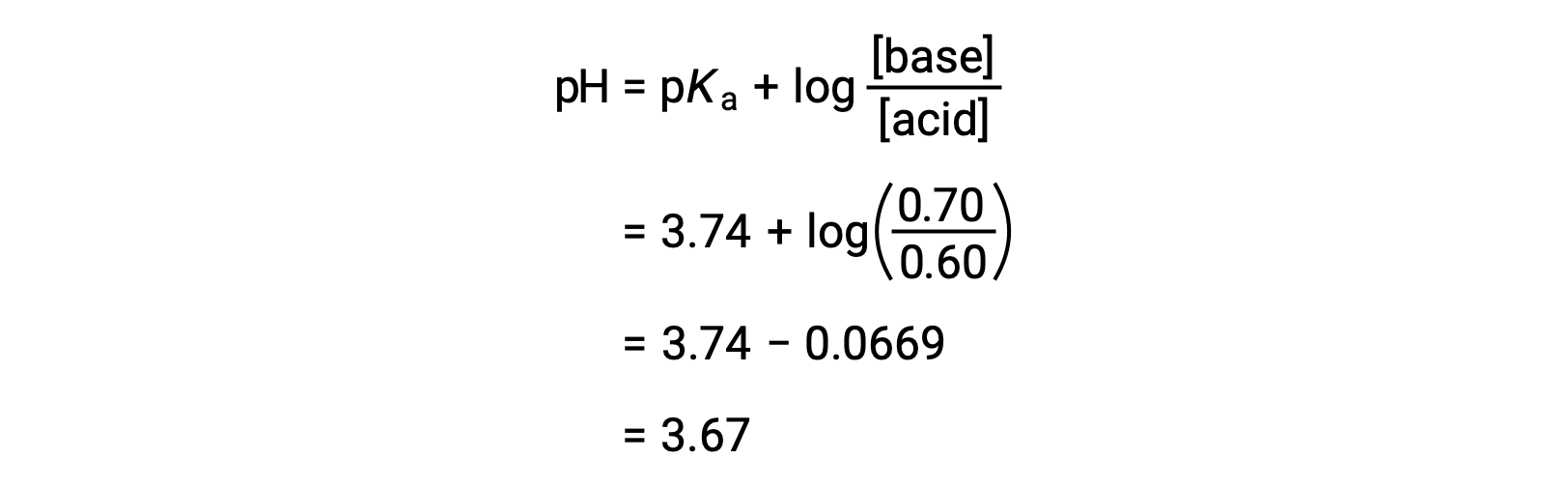

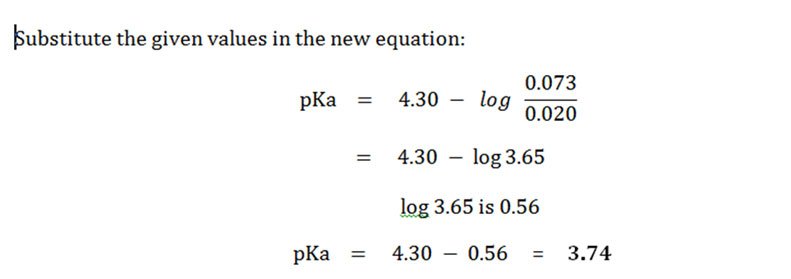
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)
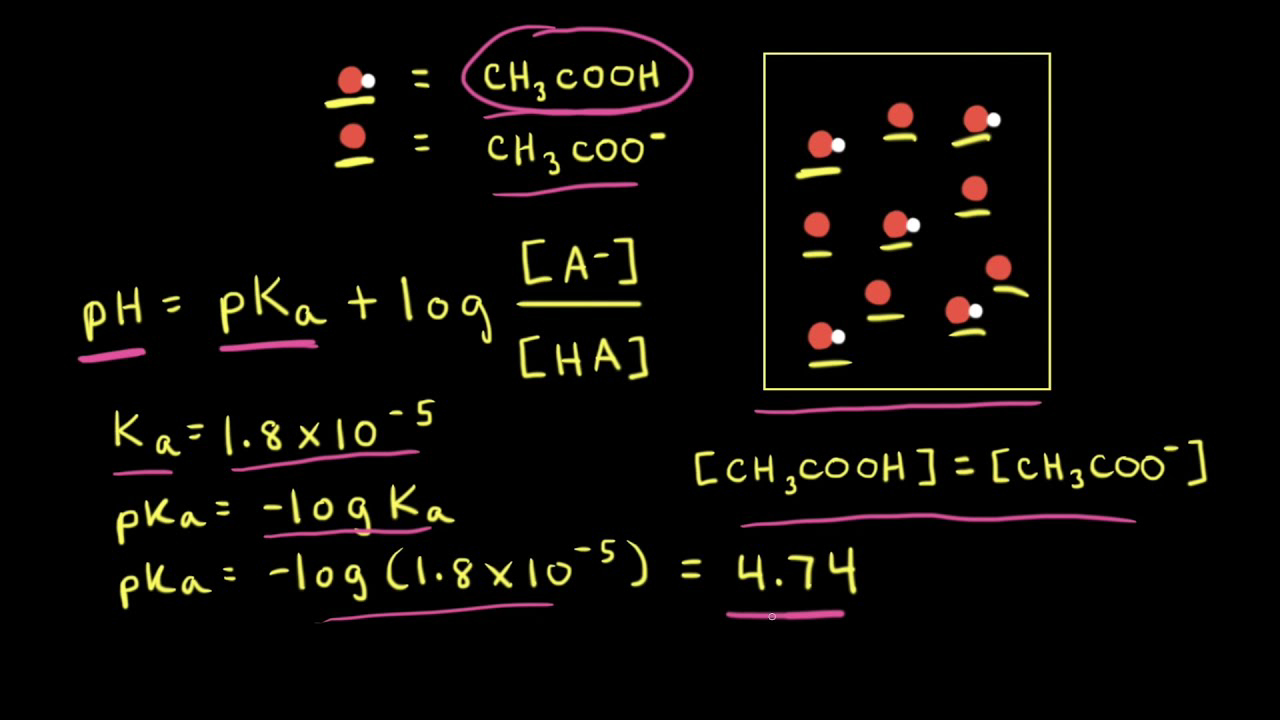
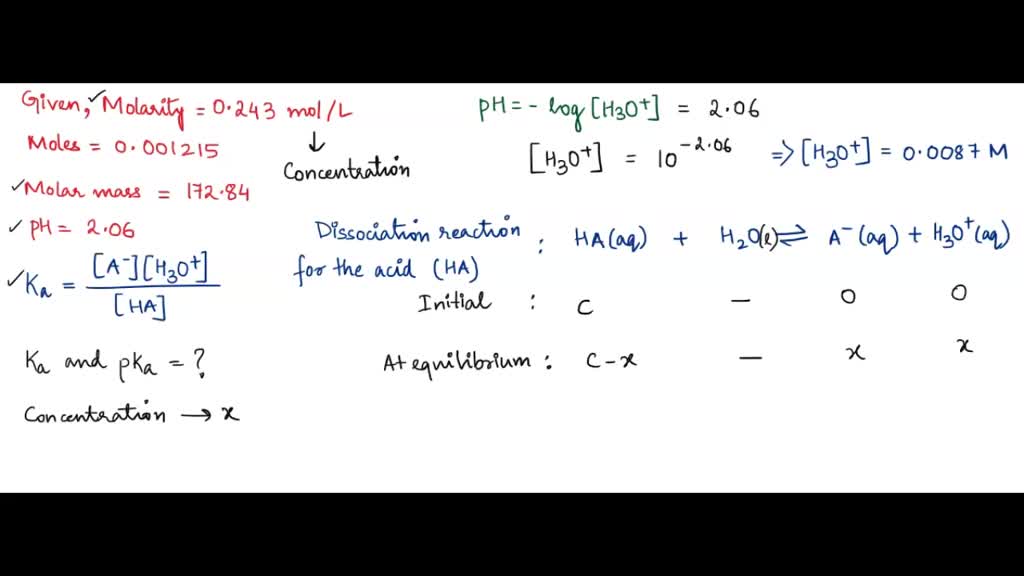
![Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com](https://media.cheggcdn.com/media/375/3755951c-9b2e-4931-a914-33bb100113d8/php72x3Cn.png)