Stability Trials ASEAN Guidelines. The Objective of a stability study To determine the shelf life, namely the time period of storage at a specified condition. - ppt download
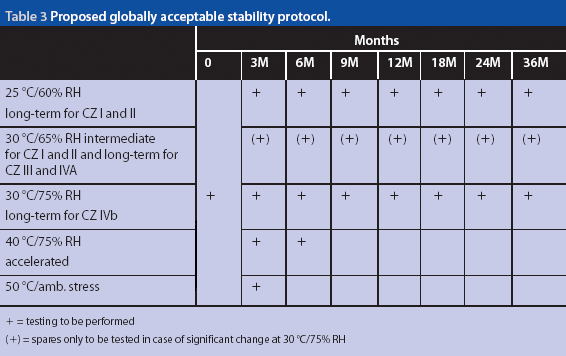
82 APPENDIX 11: GUIDELINE FOR STABILITY DATA For detailed information please refer to the ASEAN Guideline on Stability Study of

ASEAN Guideline on stability study of drug product - suppalak.ph - Page 3 | Flip PDF Online | PubHTML5

ASEAN Guideline on stability study of drug product - suppalak.ph - Page 42 | Flip PDF Online | PubHTML5

Stability Trials ASEAN Guidelines. The Objective of a stability study To determine the shelf life, namely the time period of storage at a specified condition. - ppt download

Regulatory Strategy for Long-Term Stability Conditions to Support Submission in Zone IV Countries | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
Q&A on ASEAN Stability Guideline 1. Q: Are stability testing parameters for example 'Friability' and 'Viscosity' and

Stability Trials ASEAN Guidelines. The Objective of a stability study To determine the shelf life, namely the time period of storage at a specified condition. - ppt download